
News
Vaccines made by purifying an immunogenic component of a pathogenic microorganism using physicochemical methods or by expressing that component using genetic engineering, purifying it, and adding adjuvants. This is because among the multiple specific antigenic determinants carried by macromolecular antigens, only a small number of antigenic determinants act on the protective immune response. In contrast, subunit vaccines are vaccines made by extracting, purifying, and assembling the components of the major protective antigenic determinant clusters present in pathogenic microorganisms.
Compare with whole inactivated virus vaccines , subunit vaccine is more stable, secure and effective .
High purity of antigen concentration
Great stability
Longer shelf life
High safety as no virus nucleic acid leading no infectious
Better immunogenicity, which can stimulate the body to produce a strong immune response
Lower manufacture costs
The highlight of Pulike Foot-and-mouth disease subunit vaccine is E.coli expression system technology
1.A series of technical reserves for plasmid optimization and transformation, gene sequence optimization, genetically engineered bacteria transformation, high-density fermentation, protein chromatography purification, etc.
2.Mature techniques for protein structure analysis such as in vitro assembly of VLPs, cryo-electron microscopy analysis of VLPs, crystal culture, etc.
3.In the previous stage, Pulike has realized the mass production of IBDV VP2 protein and PCV Cap protein, and the series products have been marketed and sold
Construction Strategy:Co-expression of 3 protein with one Plasmid
Expression Validation : 6 plasmids were expressed and then purified to achieve soluble expression of the target protein
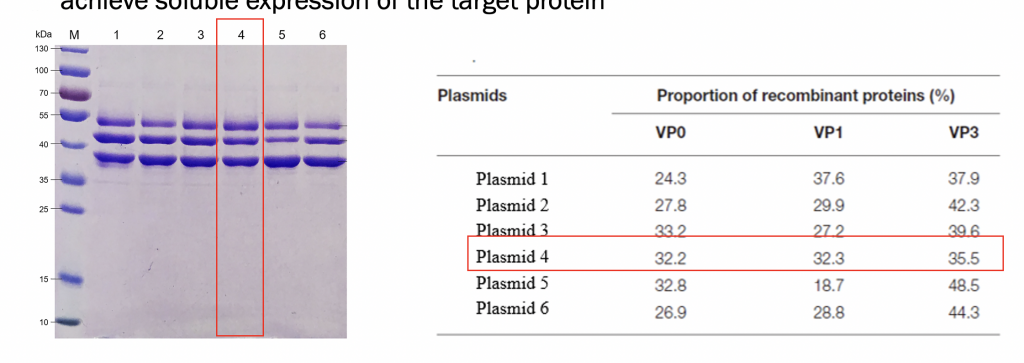
Optimization by plasmid screening to obtain strains with an expression ratio of nearly 1:1:1, laying the foundation for efficient assembly of subsequent virus-like particles(VLP)
The fermentation, purification and assembly of VLPs for large-scale production of proteins were achieved through five process steps: high-density fermentation, protein extraction, protein crude purification, protein chromatography, and VLPs assembly.




Through monoclonal strains screening, single factor condition screening of shake flasks, optimization and amplification of high-density fermentation parameters, high-density fermentation process parameters that can uniformly and efficiently express the target protein were determined.
Through the optimization of pretreatment conditions, chromatographic purification conditions, enzymatic digestion rate and assembly conditions, a purification and assembly process with high recovery and high assembly efficiency was determined, and a linear amplification of the purification process was achieved.